Sf5+ Molecular Geometry
Sf5+ Molecular Geometry. Pyramidal sqre (5.1) then it is the same as the molecular form of brf5. This compound is generally identified as being a colorless gas.
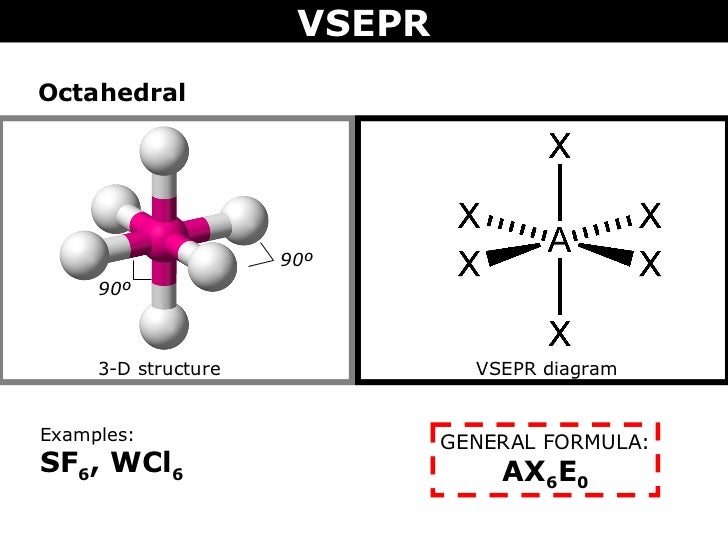
The central sulfur atom forms four bonds with the neighboring fluorine atoms and has one lone pair of electrons. Sf6 molecular geometry, lewis structure, shape, and polarity. The molecular weight of this compound is calculated to be 108.6 g/mol.
Both Molecules Have A Dipole Moment.
What is the molecular geometry of the sf − 5 s f 5 − ion? On the other hand, nitrogen dioxide, no2, is an ax2e species, and it has an angle of 134 degrees. Draw the correct lewis structure of sf4.
Sf5+ Arranges 5 Pairs Of Electrons In A Trigonal Bipyramidal Structure.
Sf5 because the molecular geometry is square pyramidal and so the bond dipole moments do not cancel out c. Sf5 what is the molecular shape of icl? The convention is to indicate the number of bonding electron pairs by the capital letter x, the number of lone electron pairs by the capital letter e, and the capital letter a for the central atom of the molecule (ax n e m).when predicting molecular geometry, keep in mind the.
The Four Groups Give A Tetrahedral Electron Geometry.
Below is the molecular geometry of sf − 5 5 −. Sf6 molecular geometry, lewis structure, shape, and polarity. Sf4 lewis structure, molecular geometry, hybridization, and mo diagram.
The Molecular Weight Of This Compound Is Calculated To Be 108.6 G/Mol.
Both molecules lack a dipole moment d. Study guides chemistry created by brielle cruickshank physics created by. The molecular shape of hci is linear.
No2 Is A Bent Molecule;
The various molecular geometries for these types of molecules are shown in tables and described on the following pages: If these are all bond pairs the molecular geometry is tetrahedral (e.g. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g.





Posting Komentar untuk "Sf5+ Molecular Geometry"